EEG Patterns in Early Childhood Differ Between Children Prone To Reward “Bad” or “Good” Actors
Abstract
Background. Early childhood is a critically important period of development for the formation of personality. Many studies provide convincing proof that elements of moral behavior are observable already in the early stages of ontogenesis. Of particular interest for psychophysiologists is the question of whether the capacity for moral evaluation in younger children can be reflected in specific EEG patterns characteristic of them.
Objective. To establish specific patterns of EEG oscillations, including the frontal alpha-rhythm asymmetry, in young children who are prone to evaluate differently the behaviors of “helping” and “hindering” puppets.
Design. Fifty-six children aged 16 to 42 months participated in the study. To measure the level of moral evaluation in children, we used the method designed by B. Kenward and M. Dahl, with some modifications. The EEG was recorded when children distributed resources among the puppet-actors.
Results. When deciding how to distribute resources among the puppets, the children with a higher moral evaluation index demonstrated an overall higher alpha rhythm amplitude, as well as a specific pattern of theta rhythm amplitude. The moral evaluation indices correlated with alpha asymmetry in the EEG loci F7 and F8.
Conclusions. 1. Significant differences in EEG patterns were found between the children who showed different levels of moral evaluation. Children with higher indices of moral evaluation showed a higher alpha rhythm amplitude when deciding how to distribute resources among the puppets, depending on the puppets’ “helping” or “hindering” behavior. 2. The theta rhythm oscillation patterns differed significantly between the samples of children with different moral evaluation indices. 3. Alpha asymmetry in the dorsolateral prefrontal cortex (loci F7, F8) was correlated with the moral evaluation indices, indicating an increased activation in the prefrontal regions of the left hemisphere in children with a more developed understanding of moral behavior.
Received: 11.02.2019
Accepted: 15.03.2020
Themes: Psychophysiology
PDF: http://psychologyinrussia.com/volumes/pdf/2020_2/Psychology_2_2020_84-95_Orekhova.pdf
Pages: 84-95
DOI: 10.11621/pir.2020.0206
Keywords: children, early age, moral evaluation, alpha asymmetry, EEG
Introduction
Moral behavior is considered as a form of prosocial behavior. Some researchers define morality as a set of prescribed norms that reflect concern for the well-being, rights, fairness, and justice of other people (Dahl & Killen, 2018; Turiel, 2015). Early childhood is seen as a period of development critically important for the formation of personality. Morality builds up through the processes of social interaction, self-reflection, and making judgments. Peer interaction plays a key role because it allows children to experience conflicts that make them reflect, summarize, and form an assessment of their daily interactions. A distinctive feature of moral behavior is its intentionality, which includes an understanding of individual goals and intentions. Intentionality can be viewed in line with understanding the mental states in other people (theory of mind). The process of moral evaluation gets increasingly complicated with the development of one’s capacity to reflect upon others’ internal states. At preschool age, it is still relatively difficult for children to understand the internal states of others (Killen, 2014).
In early childhood, the individual’s motivational sphere starts developing, while definite behavioral patterns, attitudes toward other people, and elementary concepts of morality and ethics are being formed (Barsukova, 2010; Shelina, 2012). With that in mind, a considerable amount of research has recently been carried out to study the formation of moral behavior in the very early stages of ontogenesis (Decety, 2011; Güroğlu, 2011; Young, 2012). It has been demonstrated that even in infancy, children can share objects and try to console a person who looks frustrated (Dunfield, 2010; Paulus, 2012; Svetlova, 2010; Warneken, 2006).
It has been observed that 18-month-old children are already capable of providing the basic forms of instrumental help (for example, a child can pick up a fallen object to pass it to an experimenter who cannot reach it by himself), deliberately sharing resources (such as toys and other things belonging to the child), and consoling a frustrated person (Dunfield & Kuhlmeier, 2010; Paulus & Moore, 2012; Svetlova, 2010). It was discovered that one-year-old children choose more often to help other individuals in whose behavior they see prosocial tendencies (Dunfield & Kuhlmeier, 2010). Children aged between one and two years who are more prone to console others (Zahn-Waxler, 1992) are also better at controlling their negative feelings (Hoffman, 2000).
Morality research includes studies with younger children aimed at measuring their capacity to make moral evaluations. This capacity comprises both cognitive and emotional processes and plays a key role in prosocial motivation, as well as in making decisions in response to different social stimuli (Cowell & Decety, 2015). It is a common practice to assess children’s capacity for moral evaluations within the context of fictional situations showing an interaction of “good” and “bad” actors. A child is asked to distribute a finite number of resources (such as sweets or toys) among those actors, following a demonstration of their interaction (Kenward & Dahl, 2011; Sampaio & Cabral, 2015).
In the past decade, neural mechanisms underlying prosocial and moral behaviors are drawing increased attention from researchers (Decety, 2012; de Greck, 2012; Mathur, 2010). EEG-based research is being devoted to studying the relationship between the functional asymmetry of the cerebral cortex and characteristics of moral behavior at different stages of a child’s personality development. A smaller alpha rhythm amplitude in a certain cortex region (or hemisphere) is thought to be an indicator of relatively more activity present there (Bazanova, 2012). There is evidence that the hemispheric asymmetry of the alpha rhythm amplitude in the frontal cortex can be associated with individual differences in social behavior, as well as a capacity to control one’s negative feelings both in adults (Harmon-Jones, 2004; Wheeler & Davidson, 1993) and children (Buss, 2003; Smith, 2010). A number of studies show a positive relationship between children’s moral behavior and their ability to control their negative emotions (Decety & Meyer, 2008; Moore, 2007).
It should be noted that analysis of the EEG is traditionally based on considering changes in the amplitude/power of different rhythms. It is recommended that their specific frequency ranges be defined relative to first finding the subject’s individual alpha frequency range, which is particularly important when studying children (Bazanova, 2012).
Several works have explored possible relationships between various EEG indices and prosocial behavior (Gallo, 2018) and the development of moral evaluation (Cowell, 2015), both in children and adults. However, it is not yet clear whether the level of a child’s capacity for moral evaluation might be reflected in specific EEG patterns under relevant experimental conditions.
Thus the aim of our work is to find out whether there are specific patterns of EEG oscillations, in particular frontal alpha rhythm asymmetry, in children aged about 1.5–3.5 years, depending on their capacity to morally evaluate the actions of interacting puppets. The children are divided into two samples based on their moral evaluation development. The EEG frequency ranges are be defined individually for each subject.
Basic goals of the study:
-
To compare the patterns of EEG oscillations under a sustained attention condition between the two groups of children based on their level of moral evaluation;
-
To compare the patterns of EEG oscillations in the two groups of children under condition of making a moral evaluation of puppets’ actions and distributing resources among the puppets;
-
To assess a possible relationship between the indices of prefrontal alpha asymmetry and the level of moral evaluation development in children.
Hypothesis: The level of moral evaluation development in early childhood may be reflected in specific EEG patterns in a condition of sustained visual attention and in the course of moral evaluation and distribution of resources among puppets.
Methods
The participants comprised 56 children (23 boys, 33 girls) aged 16 to 42 months. The mean age was 30.5 ± 6.5 (SD) months. The criteria for exclusion from an experimental sample were a birth weight less than 2.5 kg, known genetic disease, medical records showing CNS disorders, and clearly distinguishable left-handedness (a child prefers to use his/her left hand when manipulating objects and drawing).
To measure the moral evaluation index for each subject, we used a modified version of the method used by B. Kenward and M. Dahl (Kenward & Dahl, 2011). Each child observed two scenes enacted by three puppets (manipulated by the experimenter): “neutral”, “good”, and “evil.” Both scenes started with the “neutral” puppet climbing up stairs, but half-way up it started showing difficulties in getting to the next step (the experimenter said: “Oh, I’m so tired. Who would help me step up?”). In the first scene, the “good” puppet helped the “neutral” one to step up. In the second scene, the “bad” puppet hurt the “neutral” one by pushing it down the stairs, while the latter puppet was saying: “Oh, that hurt me so much!”). After that, the “good” and “bad” puppets were placed before the child on the table and he/she was asked to evaluate their behaviors by distributing between them five cardboard “cookies”. The individual index of moral evaluation (IME) for each child was calculated according to Table 1.
Table 1
Indices of moral evaluation (IME)
|
cookies given to the “good” puppet |
cookies given to the “bad” puppet |
Score |
cookies given to the “good” puppet |
cookies given to the “bad” puppet |
Score |
|
0 |
5 |
1 |
2 |
3 |
11 |
|
0 |
4 |
2 |
2 |
2 |
12 |
|
0 |
3 |
3 |
2 |
1 |
13 |
|
0 |
2 |
4 |
2 |
0 |
14 |
|
0 |
1 |
5 |
3 |
2 |
15 |
|
1 |
4 |
6 |
3 |
1 |
16 |
|
1 |
3 |
7 |
3 |
0 |
17 |
|
1 |
2 |
8 |
4 |
1 |
18 |
|
1 |
1 |
9 |
4 |
0 |
19 |
|
1 |
0 |
10 |
5 |
0 |
20 |
EEG was recorded in two conditions: (1) in a wakeful resting state with sustained visual attention (SVA) and (2) during moral evaluation of the puppet’s behavior (distribution of resources). To provide sustained attention, the children were asked to focus on a screen with the video of a rotating ball with a changing geometric pattern (the EEG was recorded for 20–30 seconds). This method is commonly used when registering EEG in younger children (Marshall, 2008; Orekhova, 2006). The EEG record duration when children were distributing resources depended on how much time they needed to fulfill the task. The mean record duration was 28.8 ± 16.2 seconds, with minimal and maximal values being from 10 to 74 seconds. The EEG was recorded while the children were being held on the lap of their parent.
The EEG was recorded by Mitsar-EEG-10/70-201 encephalograph with WinEEG software (Mitsar, Russia) in 16 leads: prefrontal (Fp1, Fp2), frontal (F3, F4), posterior lower frontal (F7, F8), central (C3, C4), mid-temporal (T3, T4), posterior-temporal (T5, T6), parietal (P3, P4), occipital (O1, O2). Linked earlobe electrodes were used as a reference electrode. The cutoff frequencies of high- and low-pass filters were 0.3 and 30 Hz, respectively; the EEG sampling rate was 250 Hz. The data were analyzed with the help of the WinEEG software. The EEG first underwent visual assessment to remove fragments with artifacts. For further processing, EEG segments with a duration of at least 10 seconds were divided into epochs of 2.56 seconds each. The EEG fragments were Fast-Fourier transformed with 50% overlap. The Butterworth filter with a passband of 2–25 Hz was used.
EEG parameters were calculated in individual frequency ranges. The individual alpha rhythm frequency range for each child was determined relative to the average frequency value of power spectra curve intersections when overlapping the cases of alpha synchronization and desynchronization in C3 and C4 leads. Desynchronization of the central alpha rhythm was normally observed when the child executed his/her own movements within the “perception and repetition of action” trial (not considered in this article), whereas synchronization occurred during the period of relative rest. The upper values of the theta rhythm and the lower values of the beta rhythm frequency ranges were determined relative to the individual alpha rhythm frequency range. Based on the known literature data (Marshall, 2008; Stroganova, 1999), the lower theta rhythm frequency range value was set at 3 Hz, and the upper beta rhythm frequency at 18 Hz. The amplitude values of each rhythm were log-transformed to normalize the distribution (lg, µV). Values that went beyond the three sigma limits were discarded. The alpha rhythm asymmetry values were calculated based on the amplitude values in symmetrical leads using the formula (lgА2 - lgА1), where lgА2 and lgА1 are the decimal logarithm of the amplitudes for the left and right hemispheres, respectively.
To determine the differences in EEG patterns recorded both in the SVA and resource distribution conditions, repeated-measures ANOVA was used for comparing the groups of children who have either high or low IME. To determine a possible relationship between the IME values and alpha asymmetry in the prefrontal cortex, the Pearson parametric r criterion was used. Because the log-transformed EEG amplitudes were normally distributed, the IME parameters were then also normalized using Box-Cox transformations. The differences in the children’s age (between the two IME groups) and in their IME values (between boys and girls) were estimated with the use of the t-test for independent samples.
Results
The IME values were calculated based on how the children evaluated the puppets’ actions with a mean score of 13.7 ± 4.9 (mean ± SD) points. This means that the children more often welcomed the behavior of the “good” puppet than the “bad” one. To establish specific features of EEG rhythms in children with different moral evaluation indices, the children were divided into two groups. Group 1 included 22 children, with IME below the sample mean, ranging from 1 to 13 points (8.7 ± 3.4); Group 2 included 34 children with IME above the sample mean, ranging from 14 to 20 points (17.0 ± 2.2). The mean age of children in Group 1 was 31.5 ± 5.9 months; in Group 2 it was 29.8 ± 6.8 months. There were no statistically significant differences between the groups in their age (t = 0.92, p = 0.35). In the group of boys, the average IME values were 13.6 ± 6.1 points; in the group of girls, 13.8 ± 3.9 points. No significant differences were identified either between boys and girls in their IME (t = 0.09, p = 0.92).
The EEG rhythm amplitude differences depending on the children’s IME were analyzed with the repeated measures ANOVA, with the main factors being the IME group (inter-subject factor Group) and EEG loci (intra-subject factor Locus). For the SVA condition, the main effect of the Group factor was insignificant, as well as the effect of its interaction with the Locus factor. The only significant main effect was that of the Locus factor.
Analysis of the differences in EEG amplitudes during the task of resource distribution showed a number of significant effects (Table 2).
Table 2
ANOVA results for EEG rhythm amplitudes in children based on their IME and EEG locus when distributing resources
|
Theta rhythm |
Alpha rhythm |
Beta rhythm |
||||||
|
Group |
Locus |
Group × Locus |
Group |
Locus |
Group × Locus |
Group |
Locus |
Group × Locus |
|
F(1, 26); p; ηp2 |
F(15, 390); p; ηp2 |
F(15, 390); p; ηp2 |
F(1, 29); p; ηp2 |
F(15, 435); p; ηp2 |
F(15, 435); p; ηp2 |
F(1, 27); p; ηp2 |
F(15, 405); p; ηp2 |
F(15, 405); p; ηp2 |
|
0.003 0.958 0.000 |
22.022 0.000** 0.458 |
2.153 0.007* 0.076 |
3.582 0.068 0.109 |
8.818 0.000** 0.233 |
1.744 0.040* 0.056 |
1.023 0.320 0.036 |
29.377 0.000** 0.521 |
1.400 0.143 0.049 |
Note. Group – two groups with different IME; Locus – 16 EEG loci. Significance: * р ≤ 0.05, ** р ≤ 0.001
The theta and alpha rhythm amplitudes turned out to be affected significantly by the Locus factor and its interaction with the Group factor, while the beta rhythm amplitude was only affected by the Locus factor.
The means of log-transformed amplitudes for alpha and theta rhythms in each EEG locus during the task of resource distribution among the puppets are shown in Figure 1. For the alpha rhythm, note that its amplitude in a group of children with higher IME has higher values in 14 (of 16) loci if compared with a lower IME group. The method of individual contrasts (F-statistics) showed that these differences reach statistical significance in the temporal leads (T3, T5, T6) and the central lead of the left hemisphere (C3).
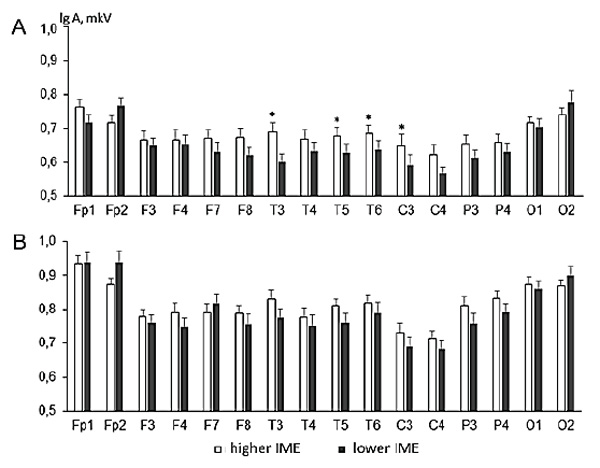
Figure 1. Alpha (fragment A) and theta (fragment B) rhythm amplitudes in children with higher IME (white columns) and lower IME (black columns) (index of moral evaluation).
Note. X-axis – EEG loci. Y-axis – mean EEG amplitudes with standard errors (lg (A), µV). Significant differences are marked by “*” for р ≤ 0.05.
Although a significant interaction effect of the Group and Locus factors for the theta rhythm amplitudes was found, the method of individual contrasts did not show significant amplitude differences in each individual EEG lead. However, we can see in Figure 1 the pattern of differences in theta rhythm amplitudes for all EEG loci in general.
It is known (Gallo, 2018; Schulte-Rűther, 2007) that prosocial behavior and moral judgement are in the first place controlled by the frontal and prefrontal cortex regions (EEG loci Fp1, Fp2, F3, F4, F7, F8). Of interest is that the left and right hemispheres contribute differently to the manifestation of prosocial behavior and moral judgments. Based on this premise, we tried to determine a possible relationship between individual IME scores and interhemispheric alpha asymmetry indices, taken as indicators of hemisphere activation and calculated for corresponding pairs of left and right frontal electrodes. For the condition of resource distribution among the puppets, significant correlations were found between IME values and frontal cortex alpha asymmetry indices for the pairs of electrodes F8/F7 (r = 0.46, p = 0.006) and Fp2/Fp1 (r = -0.34, p = 0.02). The F4/F3 asymmetry index correlated with IME non-significantly (r = 0.05, p = 0.73). The remaining pairs of electrodes (C4/C3, T4/T3, T6/T5, P4/P3, O2/O1) were also routinely checked for significant correlations;there were none. After applying the Bonferroni correction method according to the number of calculated measurements, the only still sufficiently significant (p < 0.05) correlation was that with the F8/F7 alpha rhythm amplitude ratio. The lower alpha rhythm amplitude in the left hemisphere relative to the right one in children having higher IME scores indicates that, during the process of moral evaluation, the more activated left frontal cortex is associated with a higher level of moral evaluation development in younger children.
Discussion
The aim of the present work was to study possible neural correlates of moral behavior in early childhood based on the modified method of moral understanding assessment by Kenward and Dahl (Kenward & Dahl, 2011). We examined the patterns of EEG in different experimental conditions in children having different scores for moral evaluation.
It was found that alpha and theta rhythm amplitudes differed significantly when the children were deciding how to distribute resources (cookies) among the “good” and “bad” puppets, if compared between the groups having higher and lower indices of moral evaluation. The higher IME group was characterized by a higher alpha rhythm amplitude. Taking into consideration the known role of alpha rhythm in inhibiting irrelevant signals (Bazanova & Vernon, 2014), we assume that an overall larger alpha rhythm amplitude in the higher IME group of children in the process of decision-making when distributing available resources among the actors may indicate that they possess a more optimal ratio of excitation and inhibition, higher emotional stability, and attention focus when fulfilling the task.
The topographical patterns of theta rhythm amplitude under the same condition also differed significantly between the studied groups. It is known that theta rhythm oscillations may reflect the processes of memorizing and retrieving information from memory, both in children and adults (Cuevas, Raj, & Bell, 2012). Thus, the differences we found may be associated with how the children engaged their cognitive resources when actualizing memory traces relevant to the task in regard to the puppets’ actions and deciding how to reward them afterwards.
In addition, we managed to find a significant correlation between the children’s IME score and the alpha asymmetry index in the dorsolateral prefrontal cortex (EEG loci F7, F8) when they distributed “cookies” among the puppets. Based on the idea that the level of cortex activation can be reflected in attenuated power of the alpha rhythm (Bazanova, 2011; McManis, 2002; Marshall & Meltzoff, 2011) and that a higher activation of the left frontal regions is associated with a greater ability to resist negative emotions (Mikhailova, 2017; Stroganova, 1999) and with so-called perceptual sensitivity, seen as a feature of temperament (Lobue, 2011), we presume that the more activated left prefrontal cortex underlies a more appropriate and socially acceptable evaluation of the puppets’ actions when performing moral evaluation of those observed characters.
The results of our study agree well with the conclusions made by some other researchers who demonstrated that the prefrontal cortex is involved in the process of analyzing situations that require knowledge of norms and rules (Prehn et al., 2008; Kostromina, 2017). Furthermore, the activation of the dorsolateral prefrontal cortex is associated with the ability to imagine other people’s emotional states (Decety & Moriguchi, 2007). It has been shown that an increased activation in the dorsolateral (BA 9) and frontopolar (BA 10) areas of the prefrontal cortex is registered in adults when they focus on their own emotional experiences, as well as when they try to interpret the emotions of others in photos (Schulte-Rűther, 2007). It is thought that understanding of others’ emotions (emotional empathy) is critically important in social interactions, moral decision-making, prosocial behavior, and in making moral judgments (Yodina, 2017).
To conclude, the results of our study show that children aged 16 to 42 months possess the capacity to positively evaluate moral behaviors performed by observed characters. The alpha and theta rhythm amplitude patterns differed between the groups of children who have higher or lower IME scores. The higher left prefrontal cortex region activation was characteristic of children who more often rewarded the “good” puppet. Based on these results and the conclusions of other authors, we suggest that at least during an early childhood period, children who tend to reward more often actors who behave prosocially, themselves have a more developed ability to intentionally control their own behavior and emotions. The results show that there is a neural substrate of moral behavior even in very young children.
Conclusion
-
Children who demonstrate different levels of development of moral evaluation also have different patterns of EEG when performing relevant tasks. Children with a higher index of moral evaluation have a relatively increased alpha rhythm amplitude when deciding how to distribute resources between the “good” and “bad” puppets. We assume that the larger alpha rhythm amplitudes characteristic of them under a specified condition testifies to an optimal ratio of the processes of neural excitation and inhibition, emotional stability, and attention focus when fulfilling the task.
-
The topographical patterns of theta rhythm oscillations are different in the two groups of children and may be associated with how they engage their cognitive resources in memorizing and retrieving information relevant to the task in regard to the puppets’ actions, which is needed to then decide how to distribute available resources among them.
-
Frontal alpha rhythm asymmetry during the task of resource distribution correlated with the children’s moral evaluation index. The correlation was significant for the pair of EEG loci F7 and F8, which correspond to the dorsolateral prefrontal cortex. We assume that increased activation of the left prefrontal cortex when deciding how to reward the puppets may serve as a prerequisite for the more appropriate and socially acceptable evaluation of others’ behaviors observed by younger children.
Acknowledgements
The study was funded by the Russian Foundation for Basic Research (RFBR) and the Ministry of Education, Sciences and Youth of the Republic of Crimea, under research project No. 17-415-92001 “r_a”
References
Barsukova, S.A. (2010). Problema stanovleniia sovesti v kontekste zarubezhnykh psikhologicheskikh teorii [The problem of developing a conscience in the context of foreign psychological theories]. Izvestiia Penzenskogo gosudarstvennogo pedagogicheskogo universiteta im. V.G. Belinskogo [News of the V.G. Belinsky Penza State Pedagogical University], 16(20), 179–187.
Bazanova, O.M. (2011). Sovremennaia interpretatsiia alʹfa-aktivnosti EEG [The current interpretation of EEG alpha activity]. Mezhdunarodnyi nevrologicheskii zhurnal [International Neurological Journal], 8, 96–104.
Bazanova, O.M. (2012). Alpha EEG activity depends on the individual dominant rhythm frequency. Journal of Neurotherapy: Investigations in Neuromodulation, Neurofeedback and Applied Neuroscience, 16(4), 270–284. https://doi.org/10.1080/10874208.2012.730786
Bazanova, O.M., & Vernon, D. (2014). Interpreting EEG alpha activity. Neuroscience & Biobehavioral Reviews, 44, P. 94–110. https://doi.org/10.1016/j.neubiorev.2013.05.007
Buss, K.A., Malmstadt, J.R., Dolski, I., Kalin, N.H., Goldsmith, H.H., & Davidson, R.J. (2003). Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience, 117, 11–20. https://doi.org/10.1037/0735-7044.117.1.11
Cowell, J.M., & Decety, J. (2015). The neuroscience of implicit moral evaluation and its relation to generosity in early childhood. Current Biology, 25(1), 93–97. doi:10.1016/j.cub.2014.11.002. https://doi.org/10.1016/j.cub.2014.11.002
Cuevas, K., Raj, V., & Bell, M.A. (2012). A frequency band analysis of two-year-olds’ memory processes. International Journal of Psychophysiology, 83(3), 315–322. https://doi.org/10.1016/j.ijpsycho.2011.11.009
Dahl, A., & Killen, M. (2018). Moral reasoning: Theory and research in developmental science. In J. Wixted (Ed.), The Steven’s handbook of experimental psychology and cognitive neuroscience, Vol. 3: Developmental and social psychology(S. Ghetti, Vol. Ed.), 4th edition. New York: Wiley. https://doi.org/10.1002/9781119170174.epcn410
Decety, J., & Meyer, M. (2008). From emotion resonance to empathic understanding: A social developmental neuroscience approach. Developmental Psychopathology, 20, 1053–1080. https://doi.org/10.1017/S0954579408000503
Decety, J., Michalska, K.J., & Kinzler, K.D. (2012). The contribution of emotion and cognition to moral sensitivity: A neurodevelopmental study. Cerebral Cortex, 22(1), 209–20. https://doi.org/10.1093/cercor/bhr111
Decety, J., & Moriaguchi, Y. (2007). The empathic brain and its dysfunction in psychiatric populations: Implications for intervention across different clinical conditions. BioPsychoSocial Medicine, 1(22), (PubMed: 18021398). https://doi.org/10.1186/1751-0759-1-22
de Greck, M., Wang, G., Yang, X., Wang, X., & Northoff, G. (2012). Neural substrates underlying intentional empathy. Social Cognitive Affective Neuroscience, 7, 135–144. https://doi.org/10.1093/scan/nsq093.
Dunfield, K.A., & Kuhlmeier, V.A. (2010). Intention-mediated selective helping in infancy. Psychological Science, 21, 523–527. https://doi.org/10.1177/0956797610364119
Gallo, S., Paracampo, R., Müller-Pinzler, L., Severo, M., Blömer, L., Fernandes-Henriques, C., …Gazzola, V. (2018). The causal role of the somatosensory cortex in prosocial behaviour. eLife, 7, e32740. https://doi.org/10.7554/eLife.32740.002
Güroğlu, B., van den Bos, W., van Dijk, E., Rombouts, S.A.R.B., & Crone, E.A. (2011).Dissociable brain networks involved in development of fairness considerations: understanding intentionality behind unfairness. NeuroImage, 57, 634–641. https://doi.org/10.1016/j.neuroimage.2011.04.032
Harmon-Jones, E., Vaughn-Scott, K., Mohr, S., Sigelman, J., & Harmon-Jones, C. (2004). The effect of manipulated sympathy and anger on left and right frontal cortical activity. Emotion, 4, 95–101. https://doi.org/10.1037/1528-3542.4.1.95
Hoffman, M. L. (2000). Empathy and moral development: Implications for caring and justice. New York, NY: Cambridge University Press.
Iudina, T.O. (2017). Empatiia i moralʹ: mesto vstrechi (obzor zarubezhnykh issledovanii) [Empathy and morality: Where they meet (foreign literature review)]. Shagi/Steps, 3(1), 28–39.
Kenward, B., & Dahl, M. (2011). Preschoolers distribute scarce resources according to the moral valence of recipients’ previous actions.Developmental Psychology, 47(4), 1054–1064. https://doi.org/10.1037/a0023869
Killen, M., & Rizzo, M.T. (2014). Morality, intentionality, and intergroup attitudes. Behaviour, 151(2–3), 337–359. https://doi.org/10.1163/1568539X-00003132
Kostromina, S.N., Mkrtychian, N.A., Kurmakaeva, D.M., & Gnedykh, D.S. (2017). The interrelationship between cognitive control and academic success of first-year students: An interdisciplinary study. Psychology in Russia: State of the Art, 10(4), 60–75. https://doi.org/10.11621/pir.2017.0406
LoBue, V., Coan, J.A., Thrasher, C., & DeLoache,J.S. (2011). Prefrontal asymmetry and parent-rated temperament in infants. PLoS One, 6(7), e22694. https://doi.org/10.1371/journal.pone.0022694
Marshall, P.J., & Meltzoff, A.N. (2011). Neural mirroring systems: Exploring the EEG mu rhythm in human infancy.Developmental Cognitive Neuroscience,1, 110–123. https://doi.org/10.1016/j.dcn.2010.09.001
Marshall, P.J., Reeb, B.C., & Fox, N.A. (2008). Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Developmental Psychopathology, 20(3), 861–880. https://doi.org/10.1017/S0954579408000412
Mathur, V.A., Harada, T., Lipke, T., & Chiao, J.Y. (2010). Neural basis of extraordinary empathy and altruistic motivation. NeuroImage, 51, 1468–1475. https://doi.org/10.1016/j.neuroimage.2010.03.025
McManis, M.H., Kagan, J., Snidman, N.C., & Woodward, S.A. (2002). EEG asymmetry, power, and temperament in children. Developmental Psychobiology,41, 169–177. https://doi.org/10.1002/dev.10053
Mikhailova, A.A., Belalov, V.V., Kulichenko, A.M., Diagileva, Iu.O., Orekhova, L.S., & Pavlenko, V.B. (2017). Osobennosti fronto-parietalʹnogo gradienta i mezhpolusharnoi asimmetrii elektroentsefalogrammy u detei-sirot v rannem vozraste [The EEG frontoparietal gradient and interhemispheric asymmetry patterns in orphans at an early age]. Uchenye zapiski Krymskogo federalʹnogo universiteta imeni V. I. Vernadskogo. Biologiia. Хimiia [Transactions of the V.I. Vernadsky Crimean Federal University. Biology. Chemistry], 3(69), 166–173.
Moore, C. Understanding self and other in the second year. In C.A. Brownell & C.B. Kopp, (Eds.) (2007). Socioemotional development in the toddler years: Transitions and transformations. New York: Guilford Press, 43–65.
Orekhova, E.V., Stroganova, T.A., Posikera, I.N., & Elam, M. (2006). EEG theta rhythm in infants and preschool children. Clinical Neurophysiology, 117(5), 1047–1062. https://doi.org/10.1016/j.clinph.2005.12.027
Paulus, M., & Moore, C. (2012). Producing and understanding prosocial actions in early childhood. Advances in Child Development and Behavior, 42, 271–305. https://doi.org/10.1016/B978-0-12-394388-0.00008-3
Prehn, K., Wartenburger, I., Mériau, K., Scheibe, C., Goodenough, O.R., Villringer, A., …Heekeren, H.R. (2008). Individual differences in moral judgment competence influence neural correlates of socio-normative judgments. Social Cognitive and Affective Neuroscience, 3(1), 33–46. https://doi.org/10.1093/scan/nsm037
Sampaio, L.R., & Cabral, G.R.E. (2015). Differences in allocation patterns and in the use of distributive principles emerge from children of Brazilian parents in Brazil and in the United States. Suma Psicológica, 22(1), 2015, 19–27. https://doi.org/10.1016/j.sumpsi.2015.05.003
Schulte-Rűther, M., Markowitsch, H.J., Fink, G.R., & Piefke, M. (2007). Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: A functional magnetic resonance imaging approach to empathy. Journal of Cognitive Neuroscience, 19, 1354–1372. https://doi.org/10.1162/jocn.2007.19.8.1354
Shelina, S.L., & Mitina, O.V. (2012). Moral judgments of modern children and teenagers in Russia. Psychology in Russia: State of the Art, 5, 405–421. https://doi.org/10.11621/pir.2012.0025
Smith, C.L., & Bell, M.A. (2010). Stability in infant frontal asymmetry as a predictor of toddlerhood internalizing and externalizing behaviors. Developmental Psychobiology, 52, 158–167. https://doi.org/10.1002/dev.20427
Stroganova, T.A., Orekhova, E.V., & Posikera, I.N. (1999). EEG alpha rhythm in infants. Clinical Neurophysiology, 110, 997–1012. https://doi.org/10.1016/S1388-2457(98)00009-1
Svetlova, M., Nichols, S., & Brownell, C. (2010). Toddlers’ prosocial behavior: From instrumental to empathic to altruistic helping. Child Development,81, 1814–1827. https://doi.org/10.1111/j.1467-8624.2010.01512.x
Turiel, E. (2015). Moral development. In R.M. Lerner (Ed.), Handbook of child psychology and developmental science (pp. 484–522), Vol. 1. New York: John Wiley & Sons, Inc. https://doi.org/10.1002/9781118963418.childpsy113
Warneken, F., & Tomasello, M. (2006). Altruistic helping in human infants and young chimpanzees. Science, 311, 1301–1303. https://doi.org/10.1126/science.1121448
Wheeler, R.E., Davidson, R.J., & Tomarken, A.J. (1993). Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology,30(1), 82–89. https://doi.org/10.1111/j.1469-8986.1993.tb03207.x
Young, L., & Dungan, J. (2012). Where in the brain is morality? Everywhere and maybe nowhere. Society for Neuroscience, 7, 1–10. https://doi.org/10.1080/17470919.2011.569146
Zahn-Waxler, C., Radke-Yarrow, M., Wagner, E., & Chapman, M. (1992). Development of concern for others. Developmental Psychology, 28, 126–136. https://doi.org/10.1037/0012-1649.28.1.126
To cite this article: Orekhova, L.S., Makhin, S.A., Mikhailova, A.A., Pavlenko, V.B. (2020). EEG Patterns in Early Childhood Differ Between Children Prone To Reward “Bad” or “Good” Actors. Psychology in Russia: State of the Art, 13(2), 84-95.
The journal content is licensed with CC BY-NC “Attribution-NonCommercial” Creative Commons license.









