Task Switching in Normal Aging and Mild Cognitive Impairment: A Diffusion Model Analysis of Reaction Times
Abstract
Background. Aging is associated with decline in various cognitive functions, including task switching – the ability to shift quickly between tasks and mindsets. Previous research has shown that older adults exhibit less efficient task switching. Mathematical modeling of cognitive processes involved in switching between tasks may shed light on the sources of switching inefficiency in normal and pathological cognitive aging.
Objective. To investigate possible sources of task-switching decline in normal and pathological (mild cognitive impairment, MCI) cognitive aging using the Diffusion Model (DM).
Design. 57 young adults, 34 healthy older participants, and 5 MCI-diagnosed older participants performed the commonly used Number-Letter switching task. Reaction times (RT) and accuracy were measured and Diffusion Models were fitted to individual reaction time distribution to obtain parameters characterizing processes involved in task switching: active, controlled task-set reconfiguration; passive, automatic task-set inertia; and response caution.
Results. Older age and MCI-pathology-related effects on switching efficiency were found for RT and, partly, for accuracy. After controlling for possible age differences between the two older groups, active processes of task-set reconfiguration had a clear MCI-related deficit, while passive, automatic task-set inertia components only exhibited a general effect of aging (pathological or not). Response caution was only related to older age, with no MCI effect.
Conclusion. Effortful task-set reconfiguration is sensitive to both age and MCI pathology, while passive processes of task-set inertia dissipation is only subject to age changes. The results support the idea of different dynamics of controlled and automatic cognitive processes in normal and pathological (MCI) aging.
Received: 16.11.2019
Accepted: 24.12.2019
Themes: Clinical psychology
PDF: http://psychologyinrussia.com/volumes/pdf/2020_2/Psychology_2_2020_109-120_Velichkovsky.pdf
Pages: 109-120
DOI: 10.11621/pir.2020.0208
Keywords: cognitive aging, mild cognitive impairment, task switching, drift-diffusion model, task-set reconfiguration, task-set inertia, response caution
Introduction
Normal and pathological aging are characterized by various types of cognitive decline. A typical correlate of aging is the decline of cognitive control (or executive) functions. Cognitive control is crucial for goal-directed behavior under changing and less structured conditions (Lezak, 1982), so its decline leads to less effective adaptation. Among cognitive control functions, task switching that is responsible for cognitive flexibility (also termed “shifting” in the executive functions literature) may especially be affected by aging. Task-set switching implies the ability to quickly transit from performing one task to performing another, and is generally related to the ability to change one’s mindset (Jersild, 1927). In cognitive control studies, task-switching ability is often tested experimentally with tests involving alternation between two simple tasks, such as judging digits’ parity or deciding whether a letter is a vowel or a consonant (Rogers & Monsell, 1995).
Reduced switching efficiency has been reported both for non-pathological aging (Kray & Lindenberger, 2000; Wasylyshyn, Verhaeghen, & Sliwinski, 2011) and pathological aging (Hutchison, Balota, & Duchek, 2010). In a previous study, we showed that there are signs of less effective task switching even in young, cognitively healthy individuals with an elevated genetic risk of developing dementia (Apoe-e4 carriers; Velichkovsky, Roshchina, & Selezneva, 2015). These results, among others, indicate that exploring task-switching performance may be a promising early indicator of age-related cognitive decline. In this study, we applied the methodology of drift-diffusion models (DDM) of reaction times (RTs) for a more detailed assessment of task-switching processes in healthy controls (both young and old) and a sample of persons with mild cognitive impairment (MCI).
The DDM Approach and Task Switching
The diffusion model approach (Ratcliff, 1978) to RT in two-choice decisions analysis assumes that the processing of a task is well described as a noisy process of evidence accumulation towards a response criterion. According to this model, a stimulus acts as a source of data which is consistent with one of the response alternatives and continuous evidence accumulation acts as a basis for decision making. Stimulus processing, from this point of view, is assumed to have a constant slope (drift rate, v), and the normally distributed noise explains RT incongruence in subsequent trials. The amount of evidence needed to be accumulated before a response can be elicited is also one of the diffusion model parameters (response criterion, a). Moreover, the relative position between two response barriers at the start of the decision process can be biased towards one of the response barriers (i.e., representing an a priori bias; starting point, z). The diffusion model also considers RT as reflecting both the decision and non-decision processes; therefore, a separate parameter is presented in the diffusion model – the non-decision time (t0).
The main advantages of applying a diffusion model to RT/accuracy data are the introduction of a common metric for assessing individual performance of a task (Voss, Nagler, & Lerche, 2013) and more exhaustive data utilization (i.e., actual RT distributions for two response alternatives). Furthermore, diffusion model parameters can be mapped onto psychologically meaningful variables that are essential in a certain task processing. In the case of non-decision time (t0), these corresponding cognitive processes are stimulus encoding and motor-response execution. However, in the specific task-switching paradigm, this parameter can also reflect active, effortful, voluntary task-set reconfiguration or preparation (Schmitz & Voss, 2012). Drift rate (v), which is usually interpreted as the speed of information uptake, corresponds to a later phase of information processing when a certain task-set is applied to a stimulus to generate a response. Consequently, in the task-switching context, drift rate is thought to reflect target task-set readiness and carry-over effects from previous trials – that is, passive and automatic processes of task-set inertia (Schmitz & Voss, 2012). The response criterion (a) is basically viewed as representing caution while making a decision (Ratcliff & McKoon, 2008). From the task-switching perspective, it is reasonable to suggest that response caution may increase in switch trials, as they are more resource-demanding than repeat trials (Schmitz & Voss, 2012).
Method
Sample
Ninety-six research volunteers participated in the study. They made up three groups: 57 healthy young adults (mean age = 25.5, range = 19–33, 38 female and 18 male), 34 healthy older adults (mean age = 58.5, range = 45–73, 20 female and 14 male), and 5 older participants with MCI (mean age = 73.6, range = 62–86, all female). All participants from the healthy older group were tested with Alexander Luria’s neuropsychological test battery and the Montreal Cognitive Assessment Scale (MoCA) and scored at least 26 points on the latter, indicating absence of dementia. Participants with MCI were outpatients examined at the Geriatric Psychiatry Division of the Mental Health Center (Moscow, Russia).
Experimental Task
A variant of the Number-Letter task was used (Rogers & Monsell, 1995), commonly employed to assess task-switching efficiency in various populations. The screen was divided into four quadrants and a number-letter pair was presented sequentially in each quadrant in counterclockwise order. When a pair was presented in either of the upper quadrants, the task was to classify the stimuli according to the number’s oddness. Participants were instructed to indicate whether a letter was a consonant or a vowel when a pair was presented in the two bottom quadrants. Tasks were alternated regularly and in a strict order, allowing participants endogenous cueing. The answer was given by pressing either the ‘Z’ or ‘/’ key on the keyboard, as they corresponded to the response categories (‘Z’ for odd numbers and vowel letters; ‘/’ for even numbers and consonant letters). The response–stimulus interval (RSI) was set to 500 ms, and stimuli were displayed until a response was given with a button press with the left and the right hand. There were no task pure-blocks, so only local switch costs could be estimated. The participants performed 24 training trials, preceding 128 test trials.
Procedure
Testing was run in individual sessions. Each participant read the on-screen instruction and was assisted by a session administrator. The number-letter task was always the first task in a series of executive tasks (antisaccade task and n-back task, not reported here). It was administered to an older participant immediately after mental state testing in case of a suitable result (e.g., at least 26 points in MMSE). The transition from training to test session was explicitly cued. Completion of the task took up to 8 minutes in younger adults and up to 15 minutes in elders.
Data Analyses
Trials from the training session were excluded from future analysis, as well as RT outliers from 128 test trials. We did not remove post-error trials and our outliers exclusion criterion was the presence of an observation in 95% of the most prolonged RTs, merged from all the groups. We also included data from participants with any accuracy scores, as we did not have a priori assumptions about MCI or healthy older groups’ representative accuracy scores.
We conducted two main branches of analyses, one based on RT and accuracy scores and the other upon estimated diffusion model parameters. Both were supposed to consider performance in repeat and switch trials separately, assuming that difference between representing local switch costs. In the RT/accuracy set of analyses, we averaged the performance of each participant in two types of trials and conducted a mixed 3×2 ANOVA with Group (young, older, MCI) as a between-subjects factor and Trial type (repeat, switch) as a within-subjects factor. The same was done for the estimated DM parameters. We used the fast-dmsoftware (Voss & Voss, 2007) and its implementation of the Kolmogorov-Smirnov (KS) method to estimate a separate model for each participant in each trial type. Most of the DM parameters were free to vary across conditions with the exception of the starting point, which was fixed in the middle between response barriers, and inter-trial variability of the starting point and drift rate variability, which were set to zero.
Results
Descriptive statistics for behavioral data are presented in Table 1. RT/accuracy group differences in both trial types are shown in Figure 1. Group mean estimates for diffusion model parameters in each of the trial types are shown in Figure 2. Each individual model fit was at least reasonable, and good in most cases (KS ps> 0.1).
Table 1
Descriptive statistics for RT (ms), accuracy (proportion correct), and diffusion model parameter estimates of young, healthy older, and MCI-diagnosed participants,split by the trial type.
|
|
Young |
Older Healthy |
MCI |
|||
|
Trial |
M |
SD |
M |
SD |
M |
SD |
|
Repeat |
|
|
|
|
|
|
|
RT |
972 |
386 |
1276 |
439 |
2484 |
1022 |
|
Accuracy |
0.93 |
0.11 |
0.86 |
0.21 |
0.8 |
0.25 |
|
Non-decision time |
0.3 |
0.1 |
0.42 |
0.16 |
1.13 |
0.68 |
|
Drift rate |
1.97 |
1.09 |
1.22 |
0.87 |
0.86 |
0.81 |
|
Response criterion |
2.04 |
0.5 |
2.29 |
0.71 |
2.77 |
0.92 |
|
|
|
|
|
|
|
|
|
Switch |
|
|
|
|
|
|
|
RT |
1582 |
505 |
2139 |
741 |
3680 |
1078 |
|
Accuracy |
0.9 |
0.13 |
0.85 |
0.21 |
0.8 |
0.32 |
|
Non-decision time |
0.46 |
0.23 |
0.85 |
0.44 |
2.12 |
1.66 |
|
Drift rate |
1.07 |
0.51 |
0.89 |
0.73 |
0.52 |
0.49 |
|
Response criterion |
2.59 |
0.62 |
2.78 |
0.75 |
3.21 |
0.79 |
A significant main effect of Trial type was found for RT (F(1, 93) = 266.9, p< 0.001) and for accuracy (F(1, 93) = 21.9, p< 0.001), indicating longer RT and more error-prone processing in switch relative to repeat trials. The effect of Group was significant for RT (F(2, 93) = 33.17, p< 0.001), but not for accuracy F(2, 93) = 2.02, p= 0.138), reflecting the increase in RT which propagated from young adults to older adults (post Tukey hoc test;p < 0.001) and from healthy older adults to the MCI-diagnosed group (p< 0.001), and similar accuracy across groups. The two-way Group × Trial interaction was significant for RT (F(2, 93) = 6.54, p= 0.002) and only marginal for accuracy (F(2, 93) = 2.16, p= 0.12), indicating larger RT local switch costs for healthy older adults and even larger switch costs for MCI-diagnosed participants. Post hoc tests revealed no differences in accuracy between groups (p> 0.1 in all pairs).
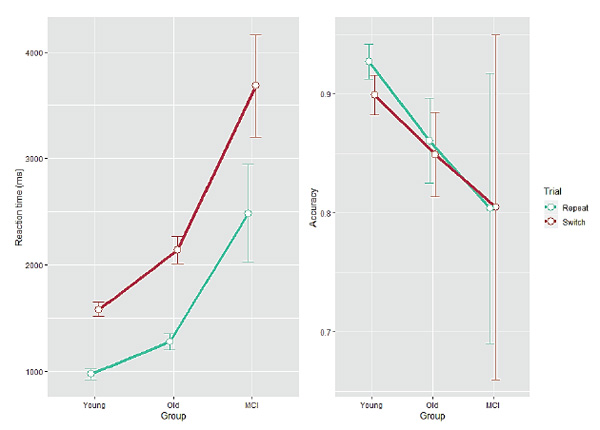
Figure 1. Reaction time (left panel, ms) and accuracy (right panel, proportion correct) as a function of Group for each Trial type. Error bars indicate one standard error from the mean.
For non-decision time (t0), we found main effects of Group (F(2, 93) = 43.96,p < 0.001), and Trial (F(1, 93) = 53.71,p < 0.001), and a significant interaction between them (F(2, 93) = 12.03,p < 0.001), indicating an increase for older participants relative to young adults (Tukey post hoc Bonferroni-corrected tests;p < 0.0005) and a steeper effect for the MCI group (as compared with the healthy older group;p < 0.0001). For drift rate (v), we found main effects of Group (F(2, 93) = 5.87,p = 0.004), Trial type (F(1, 93) = 88.97,p < 0.001), and a significant interaction (F(2, 93) = 7.87,p < 0.001). In contrast to t0, the most pronounced effects on vwere found in young adults. Post hoc tests revealed that young adults and healthy older adults differed significantly (p< 0.02), while there was no specific effect for the MCI group compared with the healthy older group (p > 0.1). For response criterion (a), we found main effects of Group (F(2, 93) = 4.19,p = 0.018) and Trial (F(1, 93) = 72.04,p < 0.001), but no interaction (F(2, 93) = 0.15,p = 0.86), indicating similar response-criterion differences in all groups. Tukey tests showed that increase of caution on the part of healthy older adults compared with young adults was marginally significant (p= 0.1) and the MCI-diagnosed participants were no more cautious than the healthy older adults (p> 0.1). However, young and MCI participants differed significantly (p< 0.03).
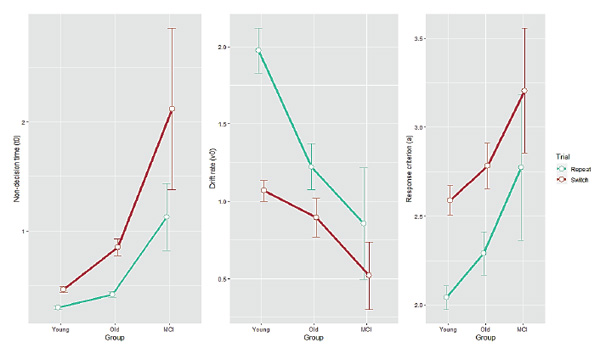
Figure 2. Diffusion model parameter estimates as a function of Group for each Trial type. Non-decision time (t0), drift rate (v) and response criterion (a) are shown in the left, middle and right panel, respectively. Error bars indicate one standard error from the mean.
As the healthy older group and the MCI group differed significantly by age (the MCI group being older,p < 0.05), the obtained effects may be due to normal, non-pathological aging processes, which may occur in MCI-diagnosed people along with many other pathological processes. To address this issue, we performed an additional analysis, statistically controlling for possible age differences. To this end, we regressed each dependent variable (RT, accuracy, t0, v, a) on age (simple linear regression) in all three groups and repeated the above analysis on regression residuals. In so doing, we hoped to cancel out possible effects of age. We hypothesized that if there is a genuine MCI effect, the young group and the healthy older group will cease to be statistically different, but the MCI group will still differ significantly from them.
Descriptive statistics for RT and accuracy after controlling for age are given in Table 2. Group mean estimates for residual RT and accuracy in each of the trial types are shown in Figure 3.
Table 2
Descriptive statistics for residual RT, accuracy, and diffusion model parameter estimates of young adults, older adults, and MCI-diagnosed participants.
|
|
Young adults |
Older adults |
MCI-diagnosed adults |
|||
|
Trial |
M |
SD |
M |
SD |
M |
SD |
|
Repeat |
|
|
|
|
|
|
|
RT |
-267.69 |
385.49 |
-624.77 |
453.7 |
286.34 |
872.85 |
|
Accuracy |
0.015 |
0.111 |
0.007 |
0.208 |
-0.023 |
0.255 |
|
Non-decision time |
-0.055 |
0.105 |
-0.359 |
0.182 |
0.161 |
0.587 |
|
Drift rate |
0.456 |
1.094 |
0.18 |
0.87 |
0.026 |
0.794 |
|
Response criterion |
-0.253 |
0.496 |
-0.315 |
0.706 |
0.029 |
0.858 |
|
|
|
|
|
|
|
|
|
Switch |
|
|
|
|
|
|
|
RT |
342.66 |
503.44 |
238.85 |
718.77 |
1483.19 |
926.83 |
|
Accuracy |
-0.013 |
0.126 |
-0.005 |
0.206 |
-0.022 |
0.326 |
|
Non-decision time |
0.112 |
0.232 |
0.072 |
0.449 |
1.149 |
1.559 |
|
Drift rate |
-0.45 |
0.514 |
-0.149 |
0.718 |
-0.31 |
0.503 |
|
Response criterion |
0.294 |
0.62 |
0.176 |
0.735 |
0.461 |
0.837 |
The same analyses as above were conducted on the residuals. For RT, a mixed ANOVA found a main effect of Group (F(2, 93) = 11.1,p < 0.001), a main effect of Trial (F(1, 93) = 266.9,p < 0.001), and a significant interaction between them (F(2, 93) = 6.54,p < 0.01). Post hoc tests revealed that there was a systematic increase in RT from the young adults through the healthy older participants (who, however, did not differ from each other,p > 0.06) to the MCI group (p< 0.001 in comparisons with both young and older healthy adults) and that switch trials were processed slower than repeat trials. Importantly, the two-way interaction was driven by the MCI group having significantly slower RTs than both the young and the healthy older groups in the switch trials. For accuracy, only a main effect of Trial was found (F(1, 93) = 21.9,p < 0.001), again indicating more errors in the switch trials. Post hoc tests revealed no differences in accuracy for either pair of groups.
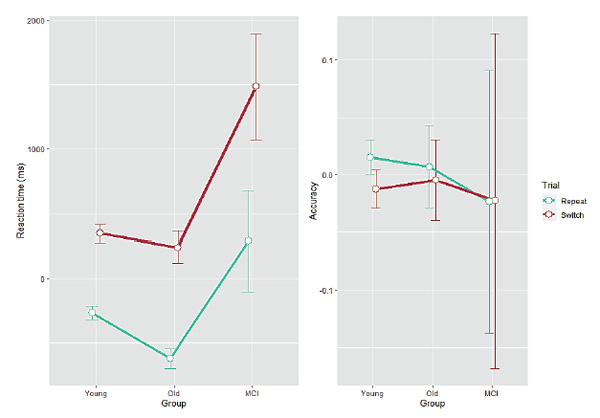
Figure 3. Residual reaction time (left panel, ms) and residual accuracy (right panel, proportion correct) as a function of Group for each Trial type. Error bars indicate one standard error from the mean.
Descriptive statistics for residual DM parameters are given in Table 2. Group mean estimates for each parameter in each of the trial types are shown in Figure 4. However, the DM parameters have lost their direct psychological interpretation (i.e., t0as time), because RTs (non-negative by definition) were not used, but rather residuals (which can be negative). So, now parameters can be negative and it is not the parameters’ values in themselves, but their relation to each other, that matters.
For the “non-decision time” (t0, which can now be negative), an ANOVA revealed all three effects: a main effect of Group (F(2, 93) = 18.1,p < 0.001), a main effect of Trial (F(1, 93) = 53.7,p < 0.001, switch trials having larger t0), and a significant interaction between them (F(2, 93) = 12.0,p < 0.001). Tukey’s post hoc test revealed that the Group effect consisted in the young and the healthy older groups forming a homogenous cluster (p> 0.05) and the MCI group differing from them with significantly higher t0 values (p< 0.001 when comparing with healthy older adults). Post hoc t-tests showed that the Group × Trial interaction was driven by a tendency of the MCI group to have higher t0in specifically switch trials. For the drift rate (v), only a main effect of Trial (F(1, 93) = 89.0,p < 0.001) and the two-way Group × Trial interaction (F(2, 93) = 7.87,p < 0.001) were found. Post hoc t-tests revealed that this interaction was quite different from the interaction obtained for residualt0. Residual drift rate did not differ between groups in switch trials after controlling for age (p> 0.05), but was significantly more negative in the young group in repeat trials (indicating quicker accumulation of information in repeat trials than in switch trials in the young group,p < 0.02). Thus, there was no genuine MCI effect for drift rate after controlling for age in switch trials, and in repeat trials the healthy older and the MCI groups still did not differ in drift rate after controlling for age (p> 0.1). For the response criterion (a), only a main effect of Trial (F(2, 93) = 72.0,p < 0.001) was found, indicating more cautious processing in switch trials. Importantly, there remained no group differences after controlling for age (p> 0.05 comparing each pair).
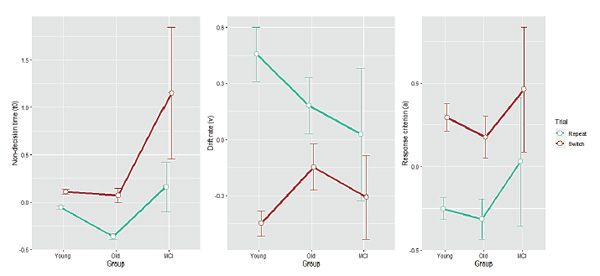
Figure 4. Diffusion-model parameter estimates as a function of Group for each Trial type. Non-decision time (t0), drift rate (v), and response criterion (a) are shown on the left, middle, and right panel, respectively. Error bars indicate one standard error from the mean.
Discussion
We compared task-switching performance in healthy young, healthy older, and MCI-diagnosed participants using a standard RT/accuracy analysis, and compared estimated parameters for a DM model of simple decision-making processes involved in task switching. We performed standard mixed ANOVAs as well as mixed ANOVAs after controlling for possible age effects.
For RT and accuracy, we found that there was a systematic increase in RTs with age, which was especially pronounced in the MCI group. Switch trials were specifically involved. This supports a considerable amount of previous results (Mayr & Kliegl, 2000; Kramer, Hahn, & Gopher, 1999), that task switching is cognitively demanding, is subject to both non-pathological and pathological aging, and may even serve as an indicator of subtle cognitive deficits in clinically healthy populations (Velichkovsky et al., 2015). Also, we found that accuracy displayed generally the same trends (larger error-related switch costs associated with advancing age and pathology) which – as typically found (Starns & Ratcliff, 2010) – were weaker, reflecting the general tendency to trade speed for accuracy in highly functioning subjects such as ours. Thus, the standard RT/accuracy analysis supported the relatively well-established findings about task-switching performance in normal and pathological cognitive aging.
Our analysis of age and MCI effects on DM parameters may be of more interest, as there are only a few such studies (e.g., Karayanidis, Whitson, Heathcote, & Michie, 2011). As explicated in the Introduction, the advantage of the analysis of DM parameters is that it allows us to find age and MCI effects on specific cognitive processes involved in the task switching. From this point of view, the most important research question for a theory of task switching in older age is whether active, controlled, endogenous processes of task-set reconfiguration (t0) are affected differently than passive, automatic, exogenous processes of task-set inertia (v, interference). We made the reasonable hypothesis that t0(task-set reconfiguration) would specifically be affected (which should result at least in a combination of a significant Group effect and a significant Group × Trial interaction, with t0increasing with age and being specifically large in the MCI group in switch trials). This is because voluntary controlled processes are typically deficient in older/demented populations (the frontal hypothesis of aging – Greenwood, 2000). We also speculated that drift rate (task-set inertia) should not be affected, as it is more an automatic process. It is a truism that automatic processes are relatively intact in both normal and pathological cognitive aging (Duong, Whitehead, Hanratty, & Chertkow, 2006; de Paula et al., 2012; Salthouse, Toth, Hancock, & Woodard, 1997).
Contrary to this, our first analysis (without controlling for age) showed that both the active and passive processes (both t0and v) are affected in a way that suggests a general aging effect and a specific MCI effect. For the task-set reconfiguration, this is perfectly understandable, and finding it was actually the goal of this study. However, the involvement of task-set inertia is more interesting. One explanation could be that task-set inertia is about the dissipation of interference from the irrelevant task. Of course, a deficit in interference control is a hallmark of cognitive aging (Hasher & Zacks, 1988), which would lead to a conclusion that the obtained effects on drift rate are driven by inhibitory deficits. However, inhibitory deficits in aging are actually deficits of voluntaryinhibition (i.e., interference control) – the most basic of the basic executive functions, according to A. Miyake and N. Friedman (2004). These may be fundamentally different from automatic interference suppression and the passive dissipation of interference which is assumed by the task-set inertia account of task switching (Allport, Styles, & Hsieh, 1994; Allport & Wylie, 2000).
Running the second analysis (after controlling for age) shed some light on this issue. The results for the active task-set reconfiguration process (t0) clearly demonstrated the predicted group differences and two-way interaction. The MCI group had systematically higher task-set reconfiguration overhead, while the healthy older subjects did not differ from the young adults in this respect (and even outperformed them numerically), after controlling for age differences. Concerning interaction, there was a specific increase in the duration of task-set reconfiguration processes in the MCI group in switch trials. This fully corresponds to our predictions. However, the task-set inertia had a different pattern of MCI-related effects – after controlling for age effects, the MCI and the healthy older group ceased to differ, while the young group had a quicker information-processing speed in repeat trials. So, if anything, there is no specific drift rate deficit associated with the MCI pathology that is different from normal cognitive aging. This suggests a differential pattern of cognitive decline in the active, controlled task-switching processes and in passive, automatic task-switching processes. This is in perfect correspondence with the general idea that controlled processes are the ones most affected in pathological cognitive aging. Automatic processes seem to decline similarly in pathological and normal cognitive aging, at least in the task-switching case.
Response criterion was the last DM parameter we analyzed. A trivial result was found that response caution is higher in the more difficult switch trials. We initially found a clear Group effect, with the healthy older group and the MCI group exhibiting more cautious processing. After correcting for age differences, the group differences vanished. This means there is no specific response caution effect associated with the MCI pathology, but a general aging effect on response caution. The effect of age on response caution has already been well documented and is sometimes thought to underlie RT slowing in older subjects (Ratcliff, Thapar, Smith, & McKoon, 2005).
Conclusions
In this paper, we fitted DM models to task switching performance data in young, healthy older, and MCI-diagnosed older participants. We found evidence that active, controlled processes of task-set reconfiguration were specifically affected by pathological cognitive aging (MCI) as well as by normal cognitive aging. For passive task-set dissipation, there was only a general effect of aging, with no specific effect of MCI pathology. Response caution was also unrelated to MCI, while it exhibited the standard aging effect. These results support the notion that controlled and automatic cognitive processes have different trajectories in normal aging, and especially in pathological cognitive aging.
Acknowledgements
This research was supported by the Russian Foundation for Basic Research under grant No. 19-013-00806.
References
Allport, A., Styles, E.A., & Hsieh, S. (1994). Shifting intentional set: Exploring the dynamic control of tasks. In C. Umiltà & M. Moscovitch (Eds.), On attention and performance 15: Conscious and nonconscious information processing.Cambridge, MA: MIT Press.
Allport, A., & Wylie, G.R. (2000). Task switching, stimulus-response bindings, and negative priming.Cambridge, MA: MIT Press.
Duong, A., Whitehead, V., Hanratty, K., & Chertkow, H. (2006). The nature of lexico-semantic processing deficits in mild cognitive impairment. Neuropsychologia, 44, 1928–1935. https://doi.org/10.1016/j.neuropsychologia.2006.01.034
Friedman, N.P., & Miyake, A. (2004). The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology: General, 18,893–900.
Greenwood, P.M. (2000). The frontal aging hypothesis evaluated. Journal of the International Neuropsychological Society, 6(6), 705–726. https://doi.org/10.1017/S1355617700666092
Hasher, L., & Zacks, R.T. (1988). Working memory, comprehension, and aging: A review and a new view. In G.H. Bower (Eds.), The psychology of learning and motivation: Advances in research and theory(pp. 193–225). San Diego, CA: Academic. https://doi.org/10.1016/S0079-7421(08)60041-9
Hutchison, K.A., Balota, D.A., & Duchek, J.M. (2010). The utility of Stroop task switching as a marker for early-stage Alzheimer's disease. Psychology of Aging, 25(3), 545–59. https://doi.org/10.1037/a0018498
Jersild, A. T. (1927). Mental set and shift. Archives of Psychology, 14, 89,81.
Karayanidis, F., Whitson, L.R., Heathcote, A., & Michie, P.T. (2011). Variability in proactive and reactive cognitive control processes across the adult lifespan. Frontiers in Psychology, 2,318. https://doi.org/10.3389/fpsyg.2011.00318
Kramer, A.F., Hahn, S., & Gopher, D. (1999). Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychologica, 101,339–378. https://doi.org/10.1016/S0001-6918(99)00011-6
Kray, J., & Lindenberger, U. (2000). Adult age differences in task switching. Psychology and Aging, 15(1), 126–147. https://doi.org/10.1037/0882-7974.15.1.126
Lezak, M.D. (1982). The problem of assessing executive functions. International Journal of Psychology, 17(2–3), 281–297. https://doi.org/10.1080/00207598208247445
Mayr, U., & Kliegl, R. (2000). Task-set switching and long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition, 26,1124–1140. https://doi.org/10.1037/0278-7393.26.5.1124
de Paula, J., Costa, D., Moraes, E., Nicolato, R., Sedo, M., & Malloy-Diniz, L. (2012). Automatic and controlled attentional processes in amnestic mild cognitive impairment: The use of a mini-verbal test. Psychology, 3,379–383. https://doi.org/10.4236/psych.2012.35053
Ratcliff, R. (1978). A theory of memory retrieval. Psychological Review, 85,59–108. https://doi.org/10.1037/0033-295X.85.2.59
Ratcliff, R., Thapar, A., Smith, P.L., & McKoon, G. (2005). Aging and response times: A comparison of sequential sampling models. In J. Duncan, P. McLeod & L. Phillips (Eds.), Measuring the mind: Speed, control and age(pp. 3–32). Oxford, England: Oxford University Press. https://doi.org/10.1093/acprof:oso/9780198566427.003.0001
Ratcliff, R., & McKoon, G. (2008). The diffusion decision model: Theory and data for two-choice decision tasks. Neural Computation, 20,873–922. https://doi.org/10.1162/neco.2008.12-06-420
Rogers, R.D., & Monsell, S. (1995). The cost of predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General, 124,207–231. https://doi.org/10.1037/0096-3445.124.2.207
Salthouse, T., Toth, J.P., Hancock, H.E., &Woodard, J.L. (1997). Controlled and automatic forms of memory and attention: Process purity and the uniqueness of age-related influences. Journal of Gerontology: Psychological Sciences, 52B,216–228. https://doi.org/10.1093/geronb/52B.5.P216
Schmitz, F., & Voss, A. (2012). Decomposing task-switching costs with the diffusion model. Journal of Experimental Psychology: Human Perception and Performance, 38(1), 222–250. https://doi.org/10.1037/a0026003
Starns, J.J., & Ratcliff, R. (2010). The effects of aging on the speed-accuracy compromise: Boundary optimality in the diffusion model. Psychology and Aging, 25(2), 377–390. https://doi.org/10.1037/a0018022
Velichkovsky, B.B., Roshchina, I.F., & Selezneva, N.D. (2015). Cognitive control and memory in healthy ApoE-e4 carriers with a family history of Alzheimer's disease.Psychology in Russia: State of the Art, 8(1), 4–13. https://doi.org/10.11621/pir.2015.0101
Voss, A., & Voss, J. (2007). Fast-dm: A free program for efficient diffusion model analysis. Behavior Research Methods, 39,767–775. https://doi.org/10.3758/BF03192967
Voss, A., Nagler, M., & Lerche, V. (2013). Diffusion models in experimental psychology: A practical introduction. Experimental Psychology, 60,385–402. https://doi.org/10.1027/1618-3169/a000218
Wasylyshyn, C., Verhaeghen, P., & Sliwinski, M.J. (2011). Aging and task switching: A meta-analysis. Psychology and Aging, 26(1), 15–20. https://doi.org/10.1037/a0020912
To cite this article: Velichkovsky, B.B., Tatarinov, D.V., Khlebnikova, A.A., Roshchina, I.F., Selezneva, N.D., Gavrilova, S.I. (2020). Task Switching in Normal Aging and Mild Cognitive Impairment: A Diffusion Model Analysis of Reaction Times. Psychology in Russia: State of the Art, 13(2), 109-120.
The journal content is licensed with CC BY-NC “Attribution-NonCommercial” Creative Commons license.









